Endotoxemia (an elevated level of endotoxin in the bloodstream) is associated with, and often present in sepsis. 1 The Alteco LPS Adsorber is an add-on therapy to treat endotoxemia in sepsis and help stabilize the patient’s hemodynamic parameters in the acute situation, when standard sepsis treatment is insufficient.

What is endotoxin?
Endotoxin (lipopolysaccharide, LPS) is the toxic part of gram-negative bacteria, present in the outer membrane of the bacteria cell wall, released when the bacterial cell disintegrates. Endotoxin is the main molecular cause of sepsis; meaning the recognition of endotoxin by immune cells is important in the pathogenesis of sepsis and septic shock. 2
Endotoxin triggers the inflammatory response
Endotoxin binds to various toll-like receptors, activating signaling cascades that cause the release of pro- and anti-inflammatory cytokines and coagulation disturbances. 3,4 This can lead to sepsis, septic shock and death.
“Endotoxin is a potent initial trigger of the inflammatory response in the immune system, activating a signaling cascade leading to release of inflammatory mediators and potentially sepsis, or even septic shock. More than half of critically ill patients “have circulating endotoxin levels >2 SDs above those detected in healthy control subjects” on the day of admission.” 5
Endotoxemia
Excessive endotoxin – endotoxemia – is associated with multiple organ dysfunction (MODS), sepsis, sepsis shock and death.
By sensing endotoxin, organ damage is induced in:
- the kidney (acute kidney injury, AKI)
- lung (acute lung injury, ALI)
- heart (negative effect on cardiac contractility)
- liver (pro-coagulant state)
- the vascular endothelium (loss of barrier function and dysregulation of vascular tone) 6
What causes endotoxemia?
Endotoxemia may originate from, for example:
- Bacterial infections (in particular gram-negative bacterial infections such as meningitis and infections of respiratory, uro-genital, or abdominal origin).
- Translocation of bacteria due to gut barrier dysfunction.
- Antibiotics, potentially liberating endotoxin from the bacterial cell-wall when killing the bacteria, which may lead to a rapid clinical deterioration for the patient. 7,8
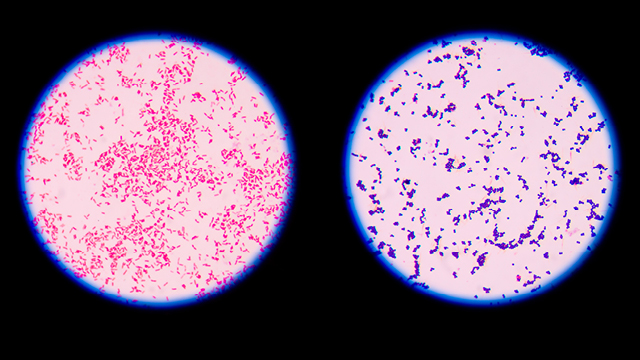
Endotoxin in gram-positive and gram-negative sepsis
Endotoxin is present in gram-negative bacteria. However, endotoxin is frequently found in the systemic circulation during sepsis, regardless of the actual microorganism found in the blood culture – endotoxemia occurs in gram-negative, gram-positive and fungal infections. 6,9
Endotoxin removal
The Alteco LPS Adsorber contains discs made of porous polyethylene (PE) covered in a tailor-made peptide. The peptide binds to lipid A (the toxic part of endotoxin) with high affinity. By adsorbing harmful levels of endotoxin from the patient’s bloodstream, the Alteco LPS Adsorber can break the chain of immune system overreactions that can spiral into septic shock. This helps to stabilize the patient’s hemodynamic parameters in the acute situation.
Webinar: endotoxin adsorption in the treatment of septic shock
Jakub Smiechowicz, Barbara Adamik
Department of Anesthesiology and Intensive Therapy
Wroclaw Medical University, Poland
Scientific articles
1 Cohen J. The immunopathogenesis of sepsis. Nature. 2002 Dec 19-26;420(6917):885-91. doi: 10.1038/nature01326. PMID: 12490963
2 M. I. Hiller, K. D. Lenz, P. A. Kocheril, C. A. Lopez, S. Gnanakaran och J. Z. Kubicek-Sutherland, ”Formulation and screening of synthetic lipoproteins for binding and inhibition of endotoxin.,” Biophysical Journa, pp. VOLUME 121, ISSUE 3, SUPPLEMENT , 2022.
3 A. Ciesielska, M. Matyjek och K. Kwiatkowska, ”TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling,” Cell Mol Life Sci. , pp. 78(4):1233-1261. , Feb 2021.
4 Jean-Marc Cavaillon, Exotoxins and endotoxins: Inducers of inflammatory cytokines, Toxicon, Volume 149, 2018, Pages 45-53, ISSN 0041-0101, https://doi.org/10.1016/j.toxicon.2017.10.016
5 John C. Marshall, et al: Diagnostic and Prognostic Implications of Endotoxemia in Critical Illness: Results of the MEDIC Study, The Journal of Infectious Diseases, Volume 190, Issue 3, 1 August 2004, Pages 527–534, https://doi.org/10.1086/422254
6 J. Smiechowicz, ”The Rationale and Current Status of Endotoxin Adsorption in the Treatment of Septic Shock,” Journal of Clinical Medicine, pp. 11, 619, 2022.
7 A. J. H. Simpson, S. M. Opal, B. J. Angus, J. M. Prins, J. E. Palardy, N. A. Parejo, W. Chaowagul, N. J. White, Differential Antibiotic-Induced Endotoxin Release in Severe Melioidosis, The Journal of Infectious Diseases, Volume 181, Issue 3, March 2000, Pages 1014–1019, https://doi.org/10.1086/315306
8 P. M. Lepper, T. K. Held, E. M. Schneider, E. Bölke, H. Gerlach och M. Trautmann, ”Clinical implications of antibiotic-induced endotoxin release in septic shock,” Intensive care medicine, pp. 28(7), 824–833, 2002.
9 Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence. 2014 Jan 1;5(1):213-8. doi: 10.4161/viru.27024. Epub 2013 Nov 5. PMID: 24193365; PMCID: PMC3916377.